Introduction
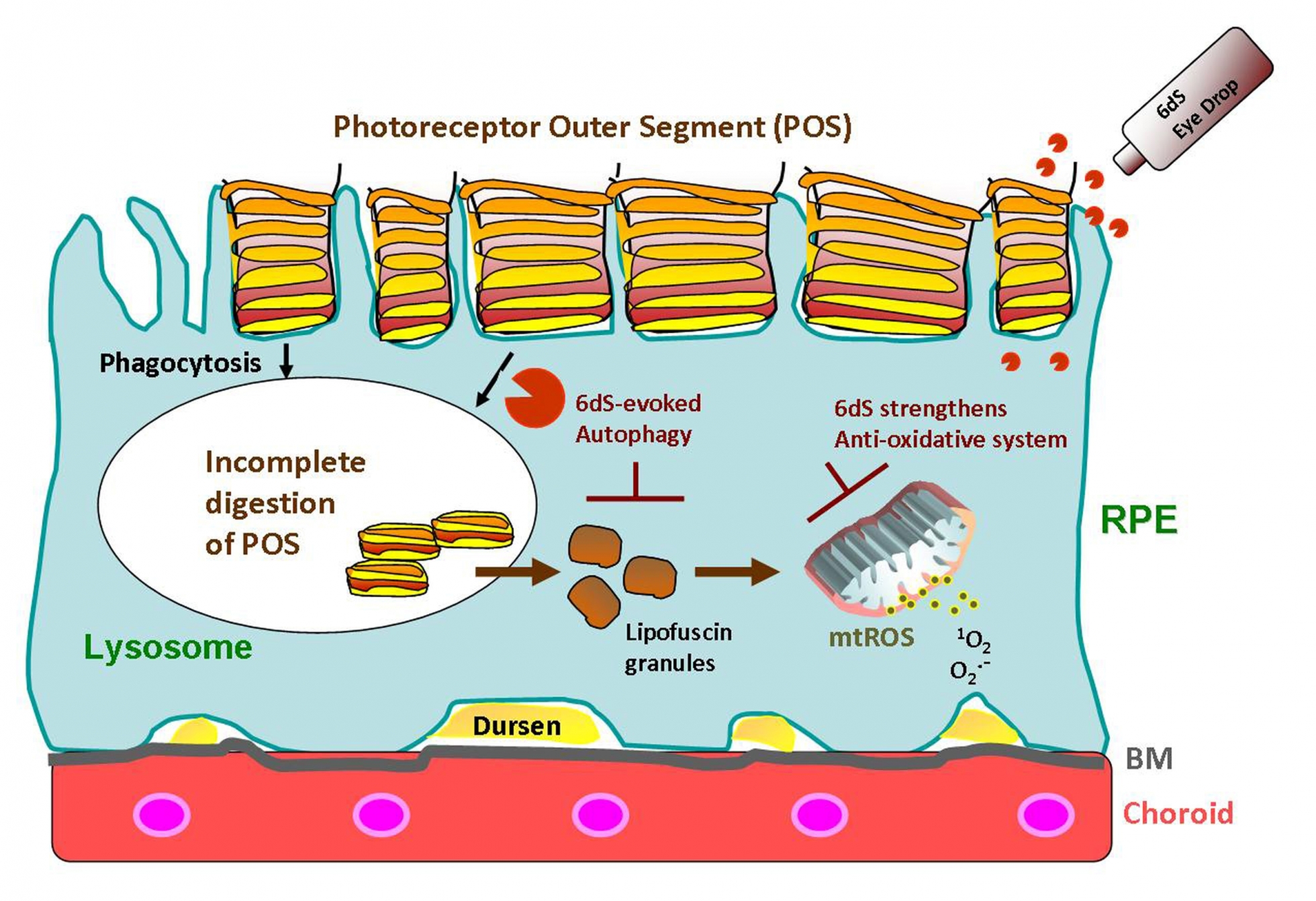
Dry age-related macular degeneration (AMD) is a blinding disease without effective treatment. In this disease, retinal pigment epithelial (RPE) cells and retinal photoreceptors are damaged by the incomplete digestion of metabolic byproduct from photoreceptor outer segments (POS), leading to lipofuscin inside RPE cells and metabolic waste under RPE, called drusen. Lipofuscin accumulation will further reduce the digestion of POS by RPE and facilitate drusen formation. Drusen prevents the exchange of metabolites and nutrient between RPE and choroid vessel and can also damage retina by its highly oxidative and inflammatory contents. ● Therefore, both of protecting RPE cells against oxidative/inflammatory stress and digesting the POS by RPE cells are important drug development direction. However, currently known pipeline drugs are still impeded these problems. ● In addition, clinical trials agents, with a few exceptions, most of the investigative therapeutics requires repeated intravitreal injection for delivery; due to the slow process of this disease, repeated and regular intraocular injections may be not suitable for slow progressing diseases.
Recently, our laboratory identified a short peptide fragment (6-mer dS; 6dS) that can offer protection of RPE cells in culture (such as NaIO3, 4-HNE and oleic acid cytotoxicity) and 6dS eye drop was shown to protect animal retina from toxic oxidant insults (such as NaIO3 and retinal ischemia/reperfusion) by evidence of histological examines and functional ERG (electroretinography). In summary, 6dS eye drop is promising to prevent/treat the deterioration of dry age-related macular degeneration.
Features / strengths
1. Fulfill unmet medical need
目前沒有藥物可用於治療 dry AMD。 6dS製成的眼藥水不會引發眼內炎,病人接受度高(由於dry AMD疾病過程緩慢,延續多年,目前開發中的藥物多需重複定期眼內注射,不適合進展緩慢疾病)。
2. First-in-Class drug with clear MOA (6dS是具有明確MOA的創新治療藥劑) -- Our drug provides a novel therapeutic strategy reliance on RPE cell protection and improving POS digestion to alleviate the pathogenesis of dry AMD.
3. Low Toxicity (低毒性) – Our drug is a short peptide derived from a human tissue protein, thus unlikely to have genuine toxicity. The wound healing effect of the 6dS on dry eye syndrome supports its anti-inflammatory and safe topical therapies.
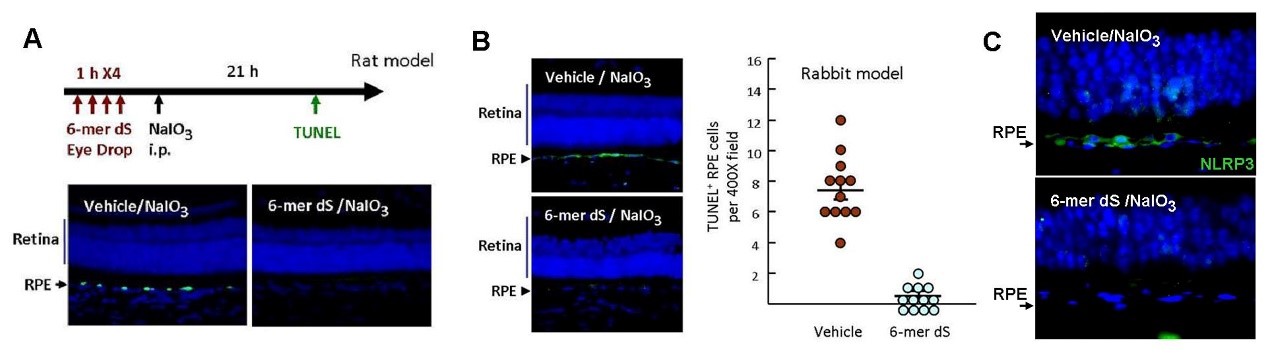
4. Pre-Clinical animal model close to human pathology (臨床前動物模型展現人類dry AMD 病理學的特徵) – The pathological features of our preclinical animal models including NaIO3-induced RPE cell death/dysfunction and I/R-induced retinal degeneration are closely similar to dry AMD. These animal models have been established by inventor’s laboratory for validation of drug bioactivity.
5. Display excellent therapeutic efficacy on dry AMD animals (用dry AMD動物模型驗證,6dS眼藥水可以保護RPE不受氧化壓力傷害,避免視網膜萎縮) -- We have demonstrated that the 6dS eye drop displays excellently effect to suppress NaIO3-induced RPE cell death (TUNEL assay) and cell dysfunction (scotopic electroretinography; ERG). In addition, our eye drop shows significantly effect to suppress ischemia/reperfusion-induced ROS and retinal damage in experimental animals.
6. In vitro model is available to estimate drug activity (體外模型可用於估計藥物活性) – In RPE cell culture, 6dS facilitates POS and lipid droplets digested by lysosomes and protects cell from cell death induced by oxidizing agents, NaIO3 and 4-HNE.
7. No further effort is required to ensure the drug synthesis & quality – 6dS can be synthesized chemically by an automated instrument abiding by GMP standard without any technical issue. The cost of manufacture is acceptable. (6dS是一種短肽衍生物,可以通過符合GMP標準的胜肽合成公司採用自動化儀器進行相關的純肽自動化化學合成,沒有任何技術問題,製造成本是在可接受的範圍)
Specification in detail
研發中
Seek of partners for business cooperation