Introduction
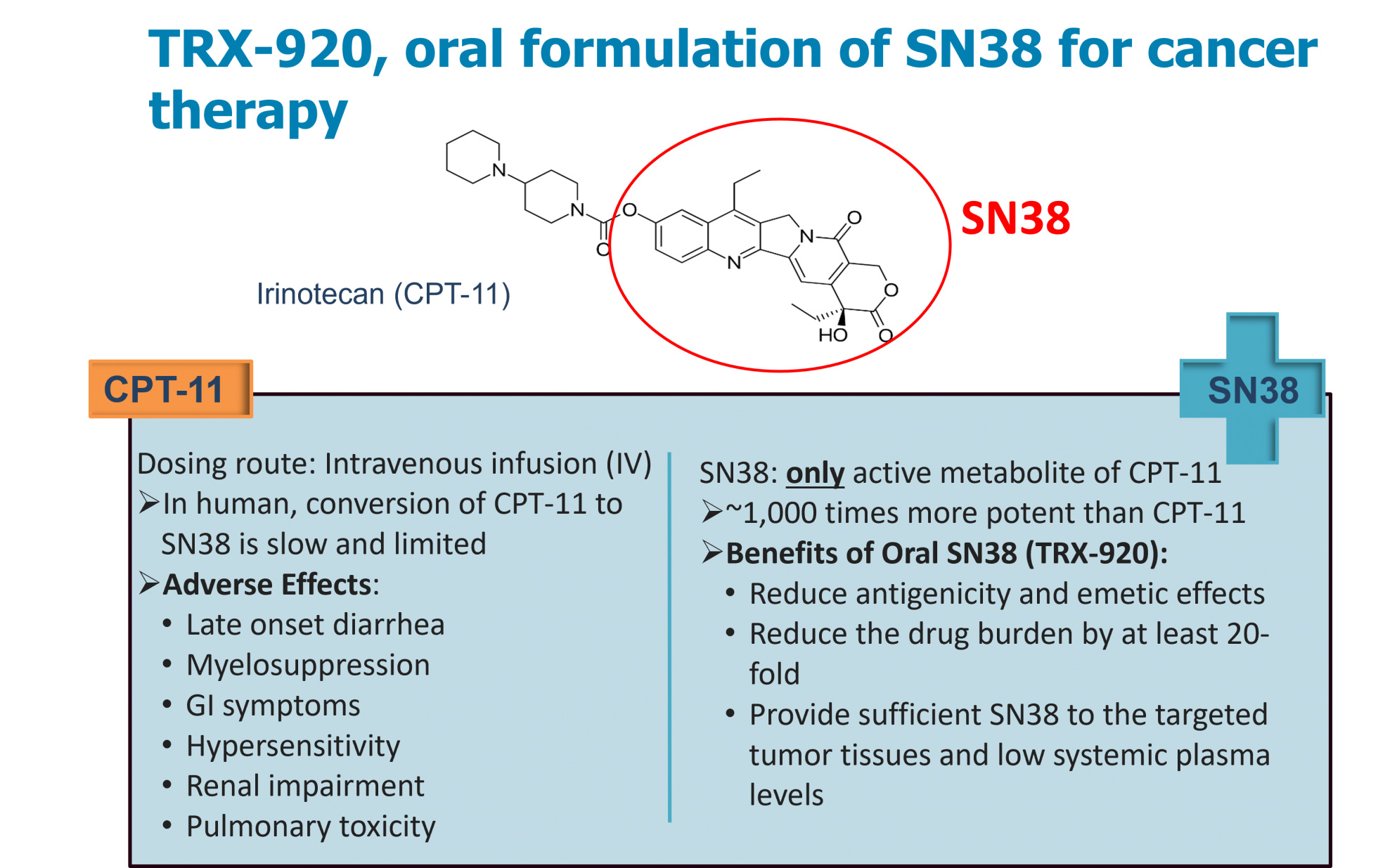
TRX-920 is an oral delivery formulation of SN38, the active metabolite of Irinotecan (CPT-11). CPT-11 is an injectable chemotherapy product approved for treating colorectal and pancreatic cancer. SN38 is ~1,000 times more potent than CPT-11 in anti-cancer activity; however, bioconversion of CPT-11 to SN38 in cancer patients is relatively slow and limited. TRX-920 is designed to has the benefit of delivering sufficient SN38 directly to the targeted tumor tissues. Oral TRX-920 is developed to reduce the burden of using high dose of CPT-11 injection without compromising overall therapeutic benefit while minimize the unwanted cytotoxic adverse effects extensively. TRX-920 is preparing for IND submission to US FDA and the clinical trial will be initiated in 2023.
Features / strengths
1. Reduce drug burden of using CPT-11 by at least 20-fold, without compromising overall therapeutic benefit
2. Reduce myelosuppression and no hematological toxicity reported for oral CPT-11
3. Patients with “hereditary fructose intolerance” can benefit from oral SN38
4. Reduce antigenicity and emesis caused by CPT-11
5. New indication potential: Liver and pancreatic cancer
Specification in detail
Formulation
Pre-filled oral syringe