Introduction
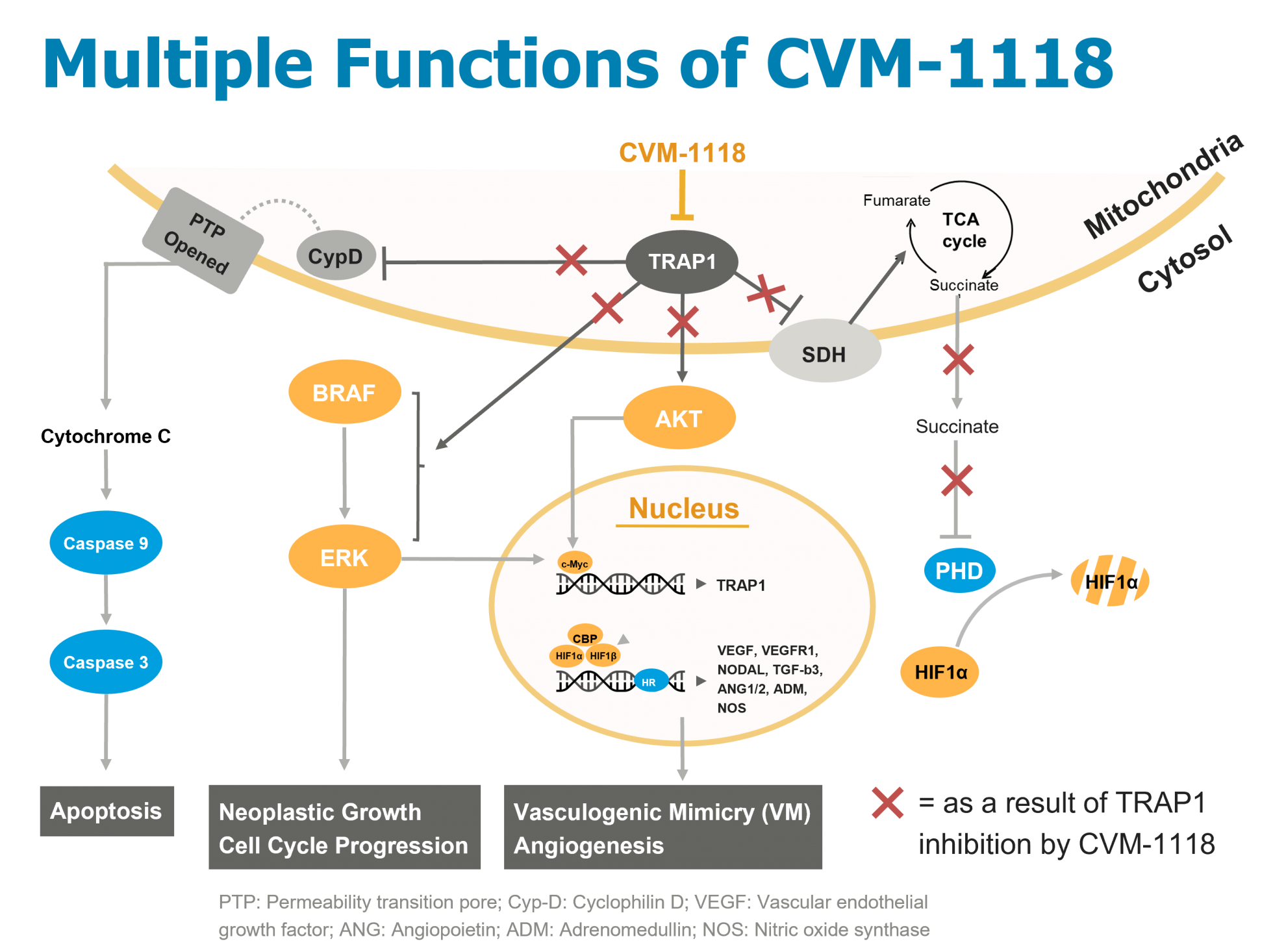
CVM-1118 is a potent new chemical entity (Foslinanib). Pre-clinical study results demonstrated that CVM-1118 has strong anti-cancer activity and favorable safety profile, with multiple and unique mechanisms of action, especially for its ability in the inhibition of vasculogenic mimicry (VM) effect. The potential target of CVM-1118 is TRAP1 (TNF receptor associate protein 1), a protein located in mitochondria, which makes it a first-in-class drug in the market. CVM-1118 showed high inhibitory effects against breast, ovarian, colon, and liver cancer in mouse xenograft models. CVM-1118, a very promising next-generation oral anti-cancer drug, is currently at clinical development stage.
CVM-1118 has been approved for Phase I safety and pharmacokinetics clinical trial by the US FDA. Two Phase IIa clinical trials are on-going for the treatment of liver cancer and neuroendocrine tumor. Extensive global patent coverage allows CVM-1118 in good IP protection for out-licensing opportunity to global pharmaceutical company.
Features / strengths
1. New molecule with first-in-class novel target (TRAP1), inhibits cancer cell growth, induces apoptosis and block metastasis by disrupting the vasculogenic mimicry (VM) formation
2. Anti-cancer effect demonstrated in multiple cancer types with IC50 <100 nM
3. Xenograft studies showed tumor growth inhibition in breast, colon, hepatoma, melanoma, and ovarian cancer models
4. High solubility and oral absorption, designed for oral administration
5. Worldwide IP protection
6. Phase II clinical trials in US and Asia (Taiwan), for HCC and neuroendocrine tumor
Specification in detail