Introduction
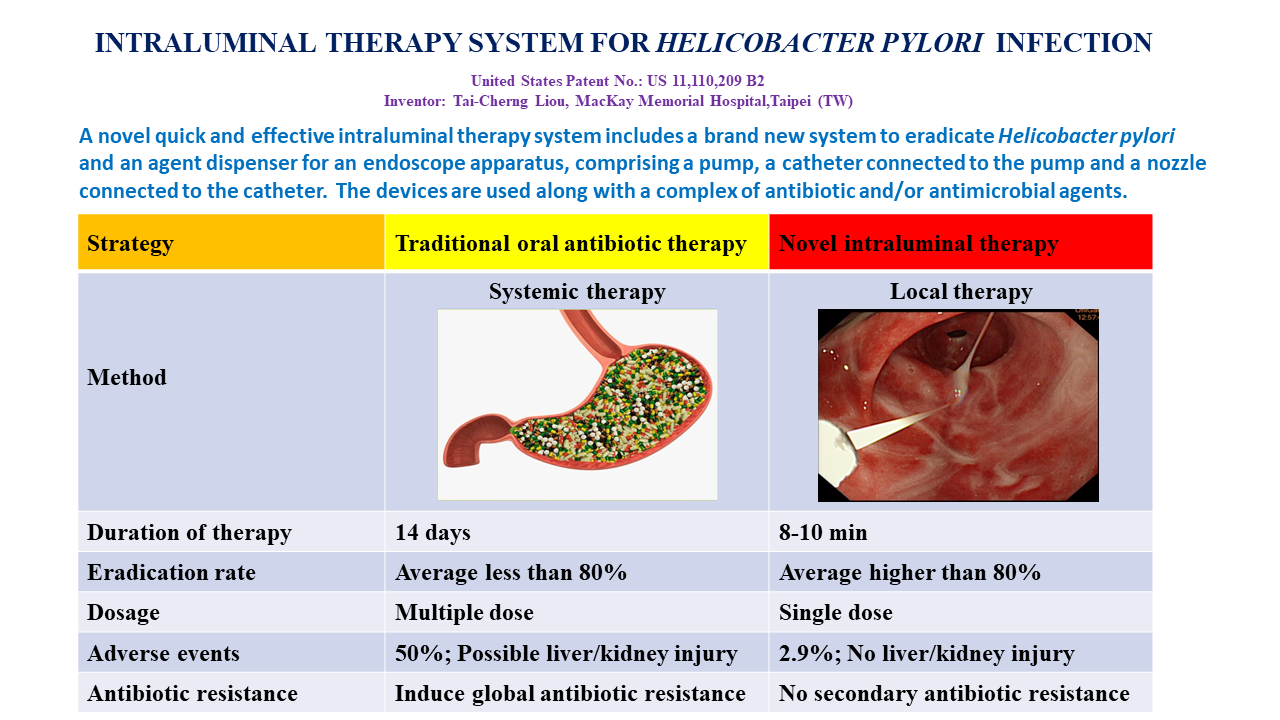
Helicobacter pylori has an infection rate of about 50% worldwide and can cause chronic gastritis, gastric and duodenal ulcers, precancerous lesions, gastric adenocarcinoma, and gastric lymphoma. Endoscopy is required to diagnose the above diseases and oral antibiotics are used to treat and prevent them.Helicobacter pylori has a unique natural barrier in the stomach: gastric acid destroys antibiotics and the gastric mucus layer protects the bacteria. This makes oral antibiotic treatment very complex and difficult, leading to an increase in drug-resistant bacterial species worldwide and a decrease in the success rate of treatment year by year. In the United States, the eradication rates of most therapeutic regimens are less than 80%. The course of antibiotic treatment lasted for two weeks, and it was very inconvenient to take high doses, multiple tablets, and multiple types of antibiotics 2 to 4 times a day. About 50% of patients will experience various side effects such as gastrointestinal discomfort, and may cause liver and kidney damage, thus interrupting treatment. In view of this, MacKay Memorial Hospital developed a novel intraluminal therapy for Helicobacter pylori infection (ILTHPI) and obtained a U.S. patent for the treatment (US11110209B2) on September 7, 2021. When patients undergo pain-reducing endoscopy, it takes 8-10 minutes to complete the sterilization treatment simultaneously. About 80% to 90% of patients will achieve immediate eradication. ILTHPI is local treatment is a local treatment and requires only one use of antibiotics; only a very small number of patients have mild side effects (2.9%), and there are no side effects on the liver or kidneys, and it will not lead to the emergence of drug-resistant bacteria.
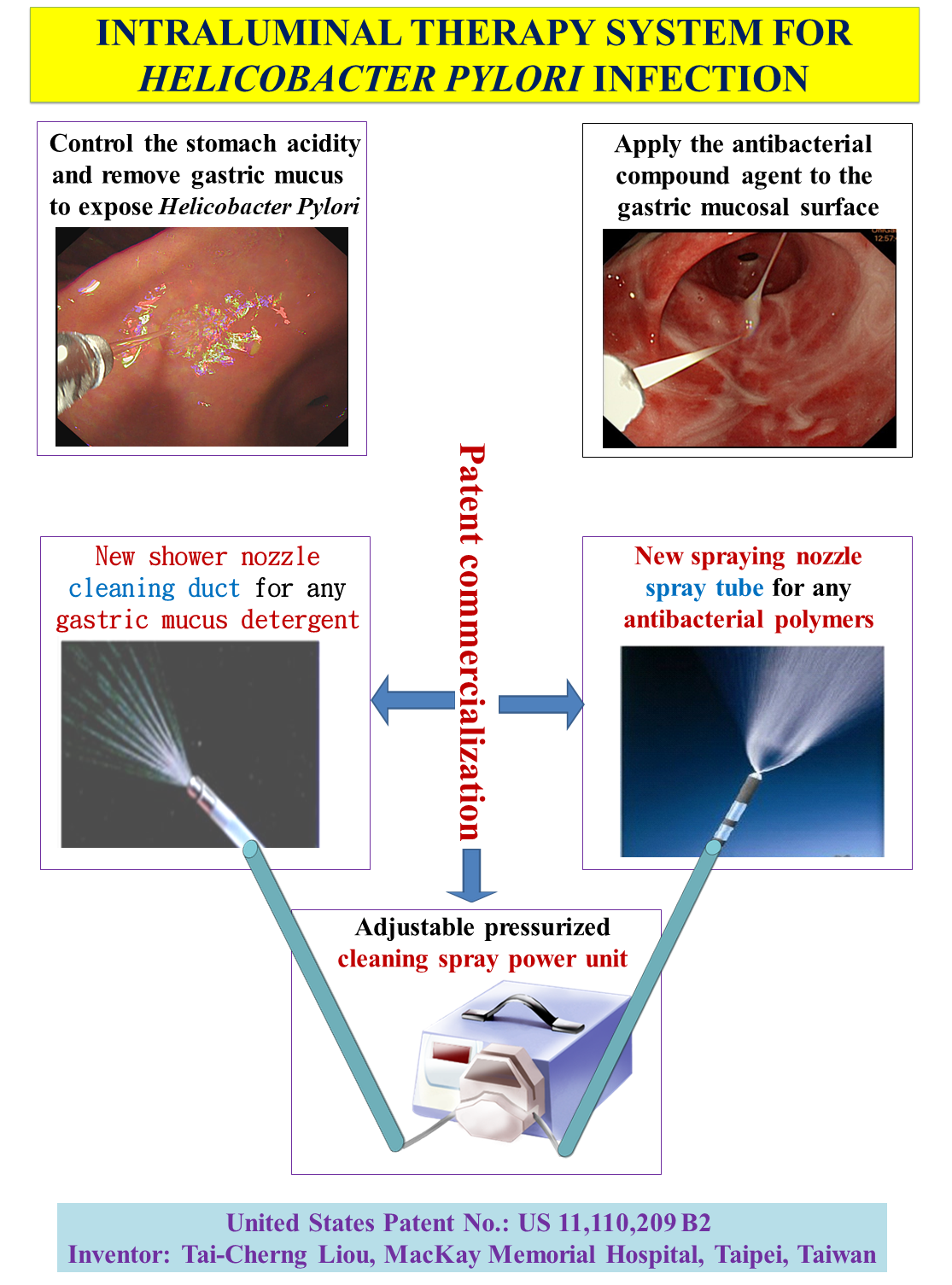
The potential commercialization value of this patent includes three parts: medicaments, catheters, and pressurization devices. Medications include various cleaning solutions and various bactericides (including antibiotic complexes or other antimicrobial sterilizing agents). The catheter includes convertible duct nozzles (shower nozzle and sprayer nozzle), and the nozzles include plastic and metal materials. The pressurizing device is a pressure-adjustable controller that
can be adjusted in multiple stages when handling different stages and purposes.
Features / strengths
A novel quick and effective intraluminal therapy system includes a brand new system to eradicate Helicobacter pylori and an agent dispenser for an endoscope apparatus, comprising a pump, a catheter connected to the pump and a nozzle connected to the catheter. The devices are used along with a complex of antibiotic and/or antimicrobial agents. (US Patent No.: US 11,110,209 B2)
Specification in detail
研發中
Seek of partners for business cooperation