Introduction
We actively develop cell therapies for various indications, aiming to provide more treatment options for patients worldwide and satisfy the unmet medical needs.
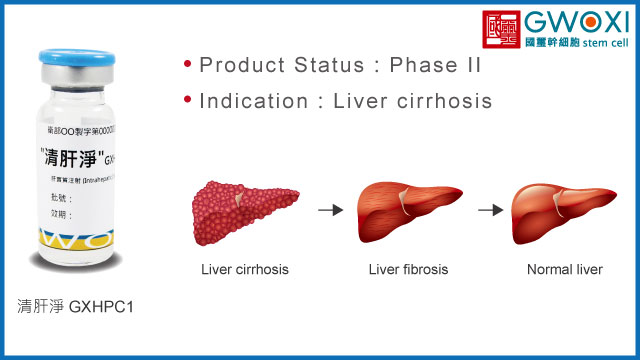
GXHPC1, applied for the treatment of liver cirrhosis, has been confirmed by preclinical trials to improve liver fibrosis by 63%, promote hepatocyte regeneration, and improve liver function indices. This research achievement has also been granted multiple international patents.
Furthermore, the results of Phase I clinical trials indicate that subjects showed improvement trends in liver function, METAVIR score, Child-Pugh score, MELD score, and quality of life, and there was no adverse event related to GXHPC1 (SUSAR) being observed, demonstrating that GXHPC1 is safe and has therapeutic potential for treating liver cirrhosis.
Features / strengths
.Advanced technology (first-in-class medicine)
.Leading stem cell medicine for treating liver cirrhosis.
.Intrahepatic (IH) injection
.The outcomes of phase I data have been published. (Cell Transplant. 2019;28(1_suppl):100S-111S. doi:10.1177/0963689719884885)
Specification in detail
Material
Adipose-derived stem cells (ADSCs)
Indication
Liver cirrhosis
Administration
Intrahepatic injection
Product Status
Phase II (NCT04088058)