Introduction
We actively develop cell therapies for various indications, aiming to provide more treatment options for patients worldwide and satisfy the unmet medical needs.
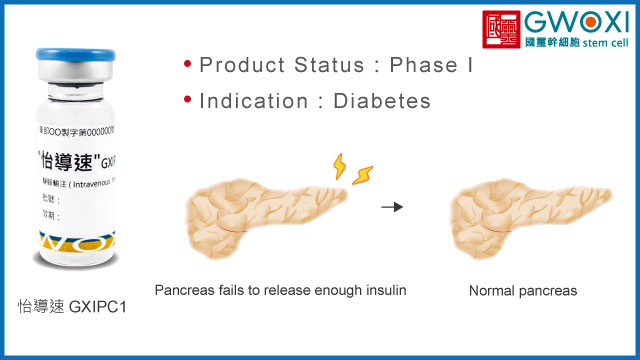
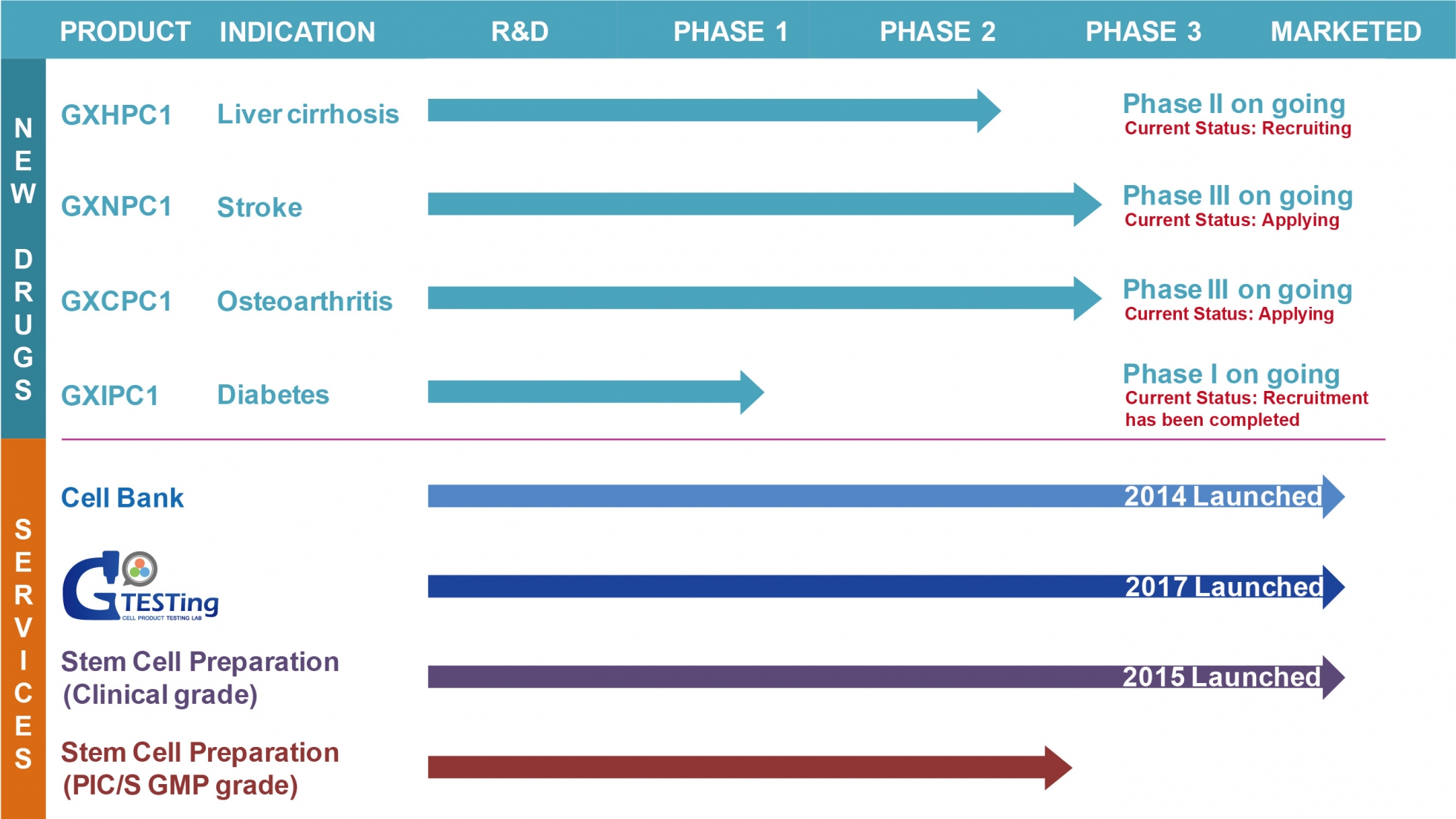
GXIPC1, used for the treatment of diabetes, has shown in preclinical study to effectively improve diabetes conditions by controlling blood sugar levels, maintaining islet cell count, and preserving islet morphology. This research results has also been granted patent.
In 2019, GXIPC1 is ongoing in clinical trial I at Vinmec Research Institute of Stem Cell and Gene Technology (VIRISG) in Vietnam. Currently, all the subjects in Phase I human trial have been recruited. This is the unprecedented case in Taiwan delivering the prepared stem cell across countries for the treatment of diabetes.
Features / strengths
.Off-the-shelf
.The allogeneic stem cell source is in compliance with the medical regulation
.GXIPC1 is a very first human trial delivering qualified allogeneic stem cell overseas in Taiwan for carrying out clinical trial
.GXIPC1 has been approved by MOH (competent authority) in Vietnam
Specification in detail
Material
Adipose-derived stem cells (ADSCs)
Indication
Type 1 Diabetes
Administration
Intravenous injection
Product Status
Phase I (NCT05308836)