Introduction
We actively develop cell therapies for various indications, aiming to provide more treatment options for patients worldwide and satisfy the unmet medical needs.
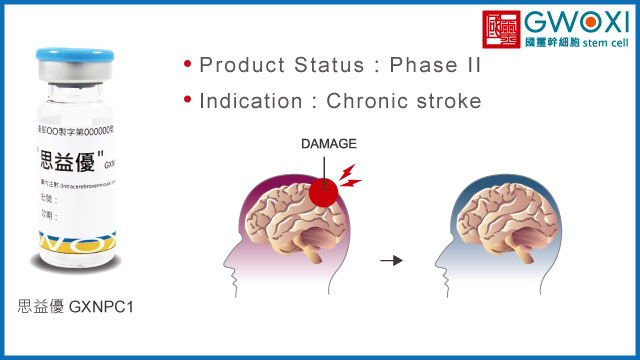
The pre-clinical study of GXNPC1 has shown the pre-conditioned stem cells can highly express the factors of angiogenesis, reducing inflammation, and inducing neural differentiation, and improve stroke-induced disabilities in mice by enhancing coordination, balance, or mobility.
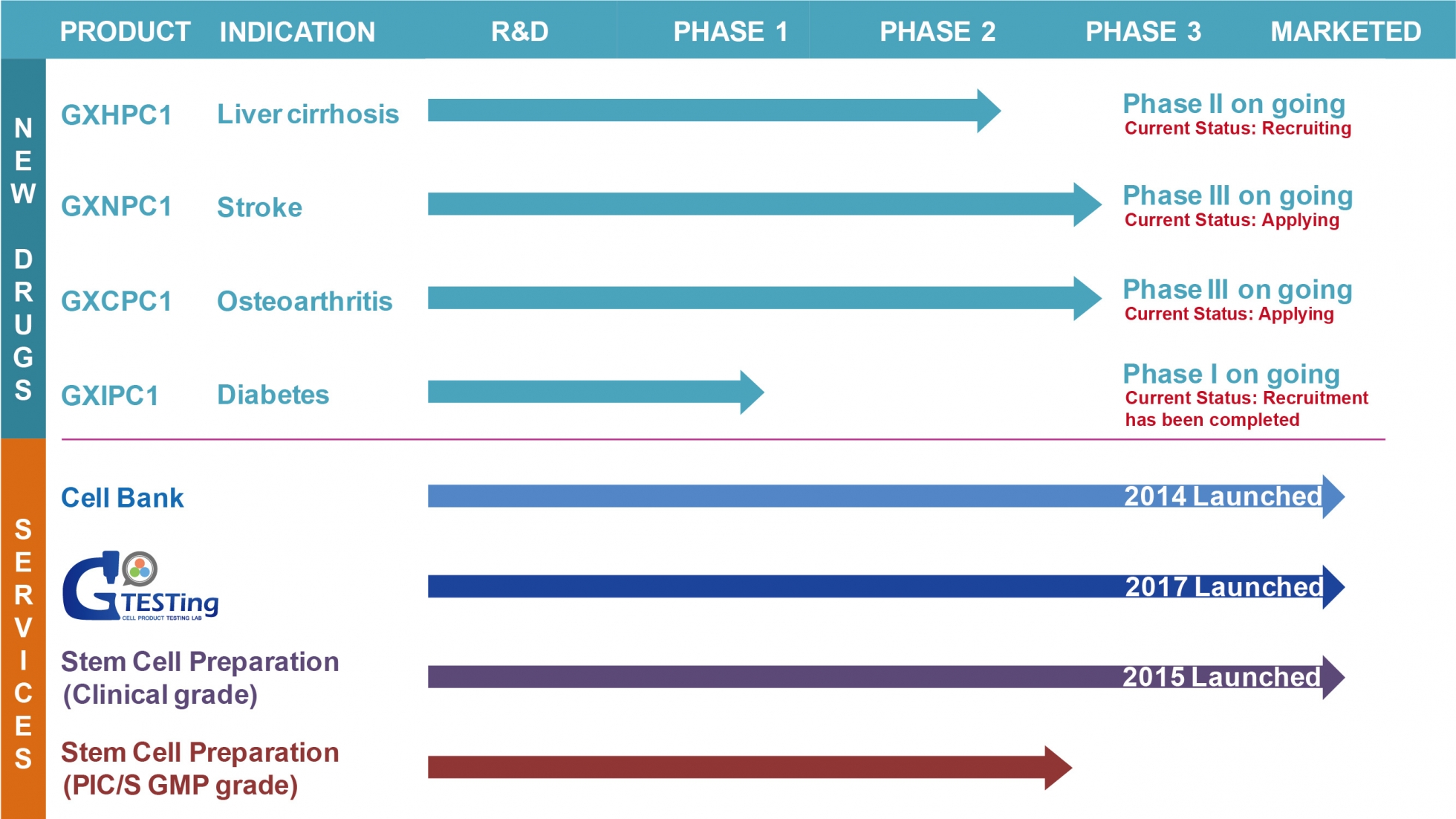
According to the GXNPC1 phase Ⅱ human trial, the outcomes showed that subjects receiving GXNPC1 treatment experienced motor recovery within two weeks. In the high-dose group, the neurological function indicator NIHSS score improved by an average of 1.2 points by the second week and continued to improve to an average of 2.7 points by the 24th week. Among them, 89% of patients showed improvement in behavior tests after treatment.
Additionally, in Phase I&II clinical trials, no safety issues related to GXNPC1 (i.e., SUSAR) were observed during the follow-up period, proving the safety of GXNPC1.
Features / strengths
.Advanced technology (first-in-class)
.Leading stem cell therapy in chronic stroke
.Intracerebroventricular (ICV) injection
.The outcomes of phase I have been published (J Tissue Eng Regen Med. 2022;16(1):3-13. doi:10.1002/term.3256)
Specification in detail
Material
Adipose-derived stem cells (ADSCs)
Indication
Chronic stroke
Administration
Intracerebroventricular injection
Product Status:
Phase III