Introduction
We actively develop cell therapies for various indications, aiming to provide more treatment options for patients worldwide and satisfy the unmet medical needs.
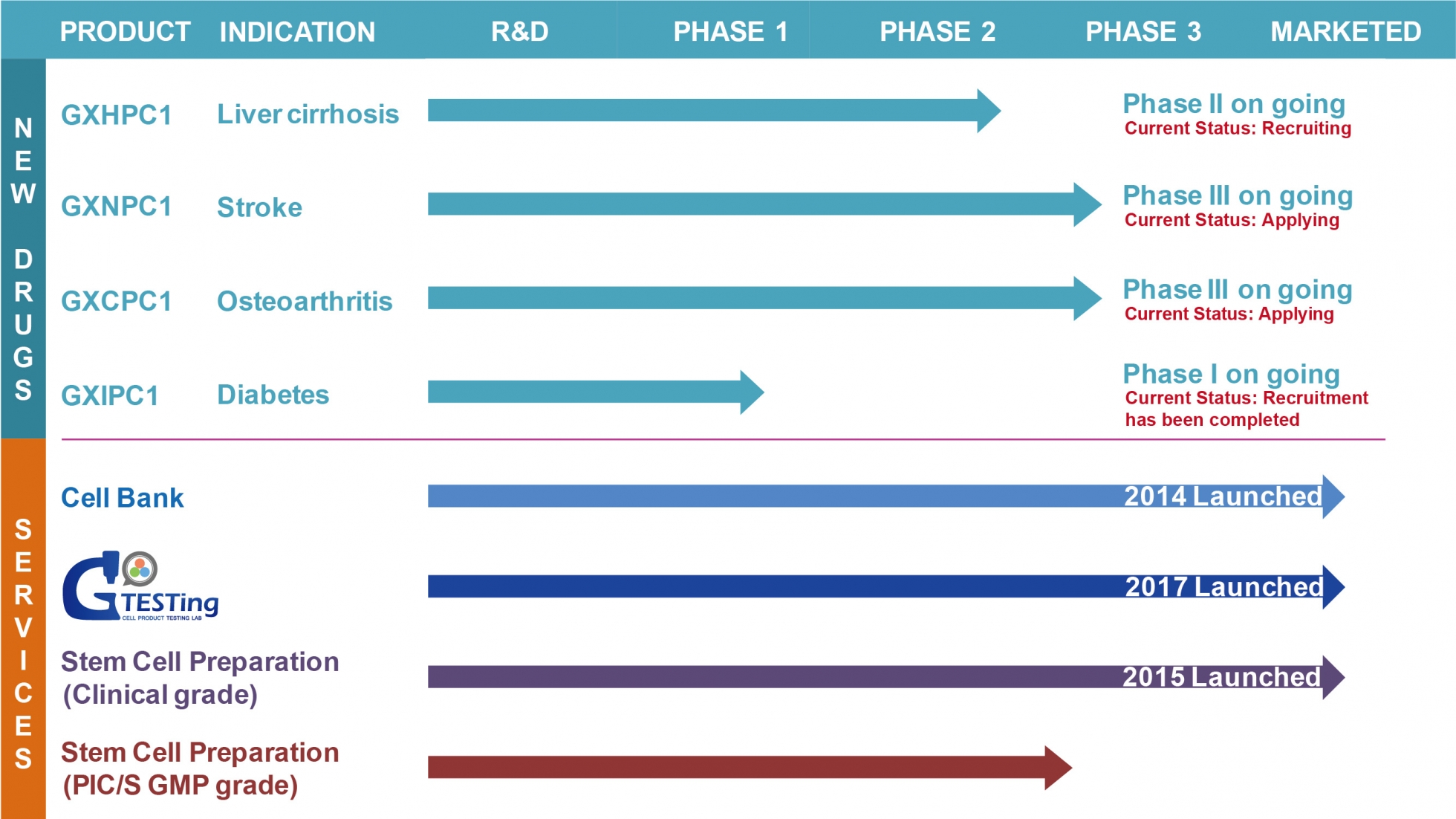
GXCPC1, used for the treatment of knee osteoarthritis (OA), has shown that it is capable of regenerating cartilage tissue in pharmacological studies. In preclinical study, histological analysis of GXCPC1 treating mice with OA-induced model revealed newly formed cartilage tissue; toxicological safety tests showed no tumor formation.
The GXCPC1 phase I&II clinical trial results demonstrate that subjects in the high-dose group (4 X 10^7 cells) improve significantly in WOMAC total score, pain, stiffness, functional subscales, pain visual analog scale (VAS), and quality of life scale SF-12. Furthermore, no safety issue related to GXCPC1 (SUSAR) was observed during the follow-up period, demonstrating GXCPC1 has the therapeutic potential for treating OA.
Features / strengths
.Allogeneic stem cell therapy
.Do not need to wait (Off-the-shelf)
.Appropriate volume for joint space (3ml)
.The allogeneic cell bank is fully complied with the medical regulation
.The outcomes of phase I&II data have been published.(Cell Transplant. 2024 Jan-Dec;33:9636897231221882. doi: 10.1177/09636897231221882.)
Specification in detail
Material
Adipose-derived stem cells (ADSCs)
Indication
Knee osteoarthritis (OA)
Administration
Intra-articular injection