Introduction
Throughout the time developing stem cell medicine, Gwo Xi Stem Cell Company has established platforms spanning the entire regenerative medicine industry chain, including pharmaceutical grade stem cell & cell derivatives preparation platform, pharmaceutical grade cell storage platform, pharmaceutical grade cell & cell derivatives product testing platform.
So far, these platforms have served several cell therapy developers, hospitals, and clinics. These services are based on our core values: Innovation, Proficiency, and Responsibility.
Each platform fully complies with the medical regulation in Taiwan. Furthermore, to maintain our services' quality, we constantly subcontract a competent third party to examine our platforms annually.
In addition, we have signed several agreements with domestic hospitals and clinics to apply stem cell therapy through special reguation in Taiwan, and we have also assisted several clinics in getting approval (100%) from Joint Commission of Taiwan. To fit our partners' need, we are commited to improving our technologies and providing comprehensive services with high quality.
Features / strengths
.Cell processing center has been approved over 10 times of GTP Inspection (100% Pass).
.Collaboration with multiple medical institutions
.Consecutively passed the SNQ for several years
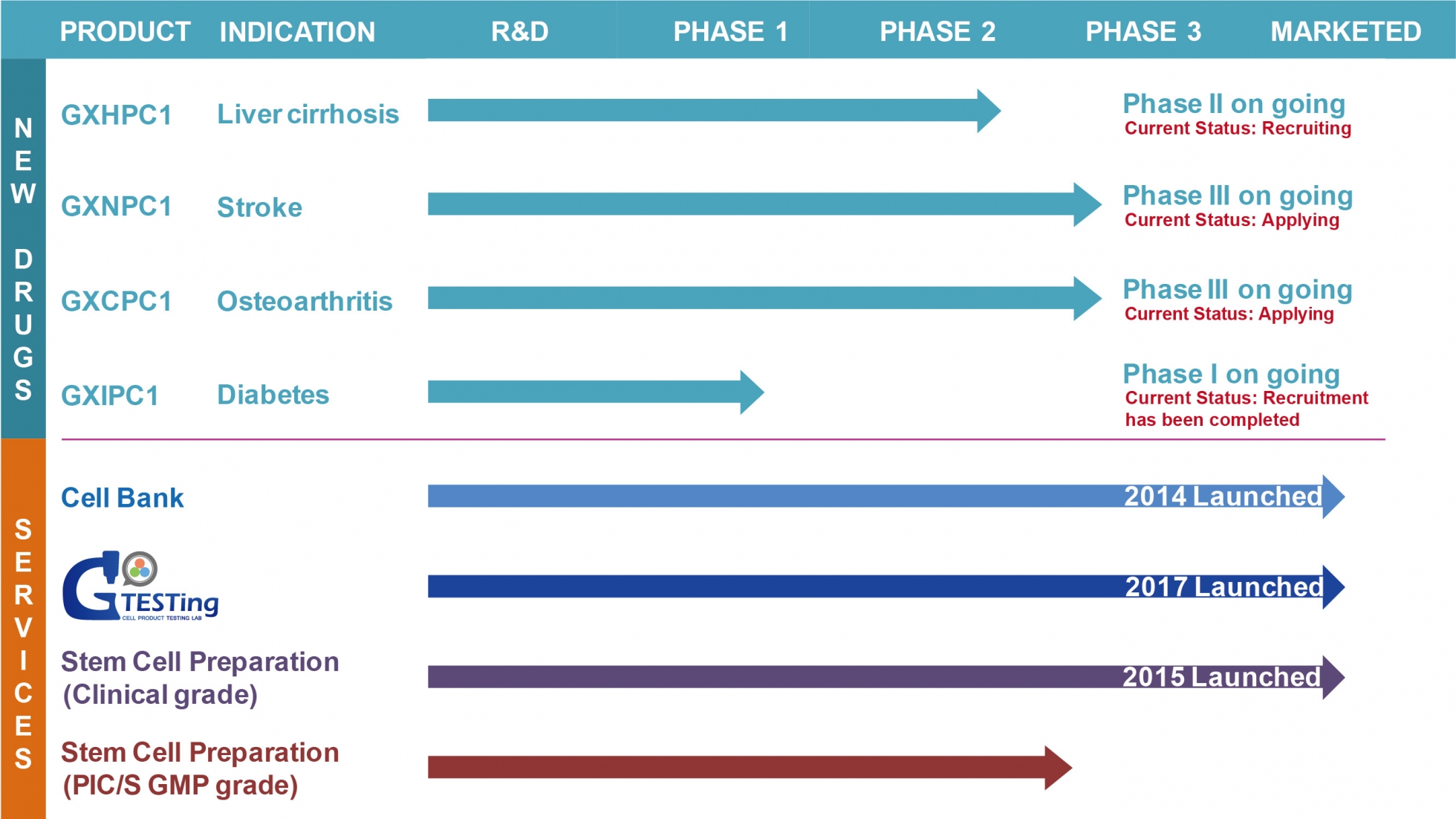
.Plentiful experience executing cell therapy clinical trial
.Four stem cell therapy medicines are ongoing in clinical trial
.Modular production design can fit the expectation from customers
.Services span the entire cell therapy industry chain
.Excuting cell therapy clinical trial overseas and managing the cold chain transport for allogeneic stem cells delivery
Specification in detail
Clinical Grade Cell Bank
Infant/Adult Cell Storage Services
Clinical Grade Stem Cell Manufacturing Platform
Applying Special Regulation for Cell Therapy (SRCT Act) in Taiwan, CDMO service
Clinical Grade Test Service for Cell Related Product
Porviding cell therapy product quality report for applying clinical trial