Introduction
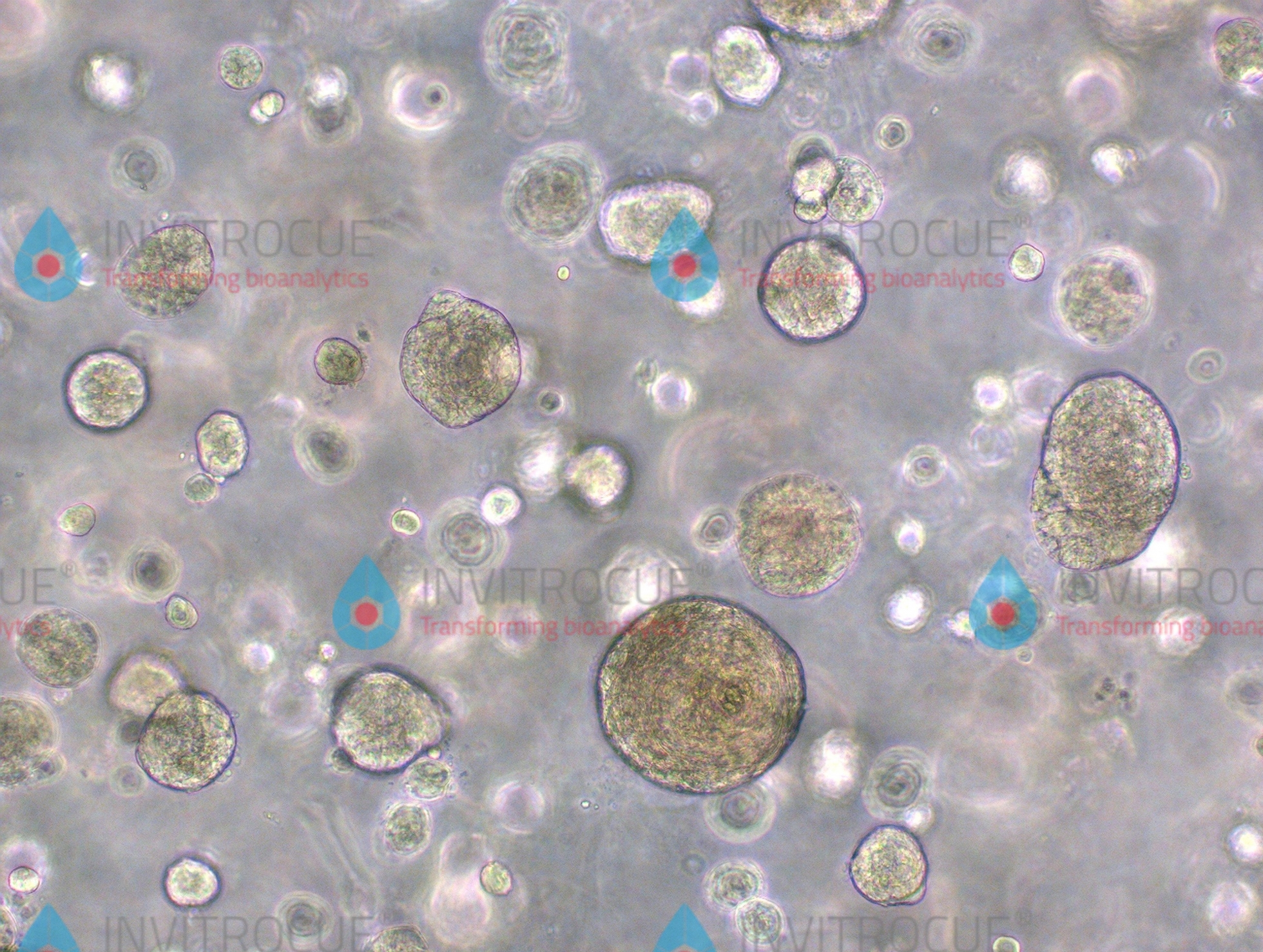
The Onco-PDO™ test has passed CE EU certification. This test uses the cancer patient's own tumor samples to replicate multiple "mini tumors" (tumor organoids) in vitro for drug testing. The attending doctor can select up to 8 designated drugs within the NCCN guidelines for testing. The test will minimize the unnecessary treatment and/or side effects that patients may undergo multiple trials of drugs. According to the Onco-PDO™ test report, the attending doctor can make a comprehensive consideration of patient’s medical condition and in vitro drug response information to select the most suitable drugs for the patient. This will save every minute and second of the treatment period, so that the patient may obtain a better therapeutic effect sooner with improved disease control and quality of life against cancer.
Features / strengths
This test provides three key clinical advantages:
• Fast turnaround: The test report is delivered within 3 weeks to ensure continuity of clinical care without delaying treatment.
• Comprehensive: The test can include all standard-of-care chemotherapeutics to ensure the most comprehensive look at the patient’s potential treatment options.
• Actionable: The Onco-PDO™ report might help oncologist to make informed treatment decisions by identifying relevant and actionable findings based upon the response of the patient’s cancer cells to chemotherapeutics in the laboratory.
Specification in detail
Certification
CE European Certification
Supporting document