Introduction
Our R&D center has been established in the Hsinchu Biomedical Science Park since 2011. We are committed to developing effective cell therapies to help the patients with unmet medical needs. Leveraging our clinical grade stem cell manufacturing platform combined with multiple proprietary technologies, we have put four stem cell medicines into clinical trial. In addition, as a forerunner, we have established stem cell factory, which will apply the PIC/S GMP certificate, in advance to put cell therapy product into mass production. To increase the stability, consistency, and scale, all our production lines were designed by adopting modular design thinking, which is involved in our GTP center and future PIC/S GMP factory. In the future, based on the solid establishment, we will stably scale up in manufacturing and, meanwhile, constantly develop innovative technologies that strengthen deployment in cell therapy industry.
Features / strengths
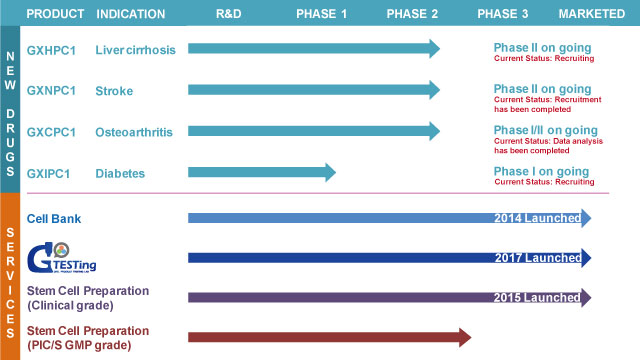
Plentiful experiences relevant to cell therapy clinical trial: Four cell therapy medicine were approved by government to execute clinical trial.
Advanced deployment: we have established cell factory in advance, which will be verified PIC/S GMP certificate.
Overseas cell delivery: we successfully execute an unprecedented clinical trial that delivered qualified allogeneic cell therapy product overseas and gained approval from MOHW in Vietnam.
Modular production line: Reducing repetitive investment, flexibly altering production, accelerating development through well-established factory.
Leveraging the achievements mentioned above, we are confident to provide our customers (either domestic or foreign), the best partnership
Specification in detail
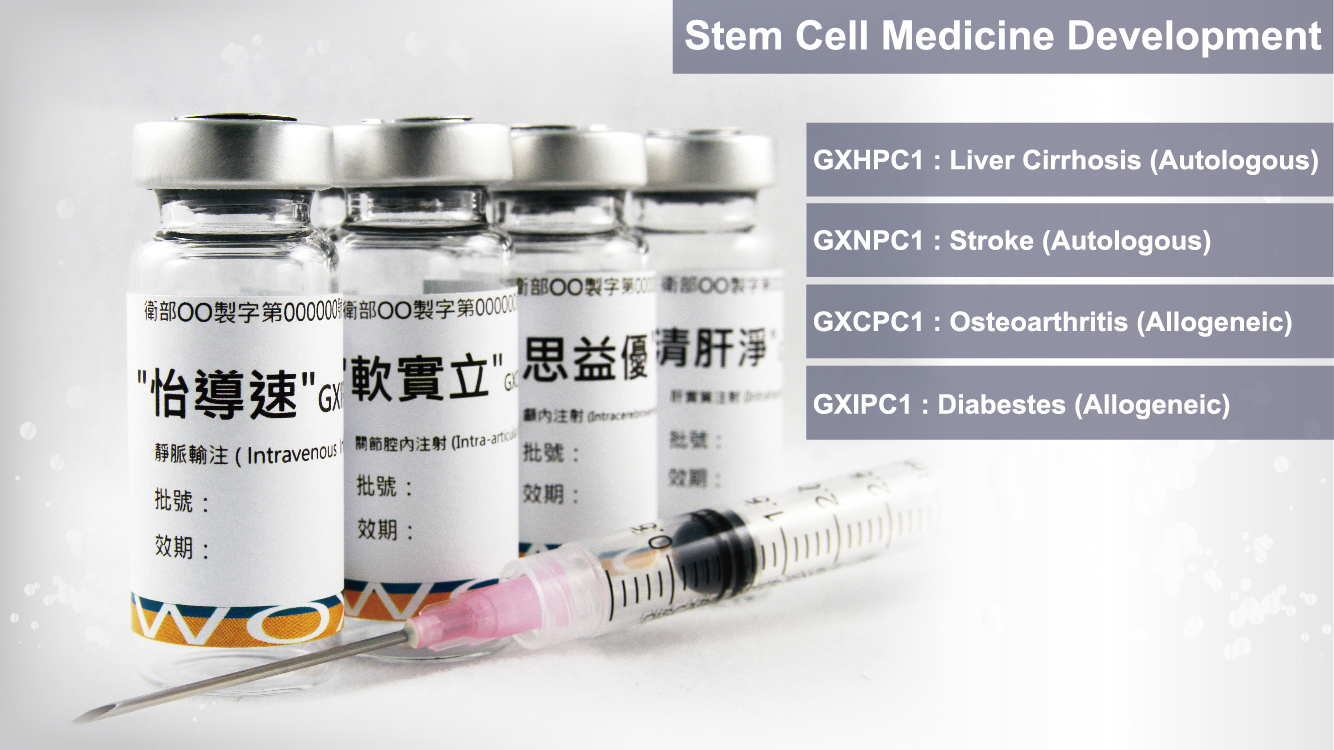
GXHPC1
Indication: Liver Cirrhosis
GXHPC1
Indication: Chronic Stroke
GXCPC1
Indication:Osteoarthritis (OA)
GXIPC1
Indication: Diabetes