Introduction
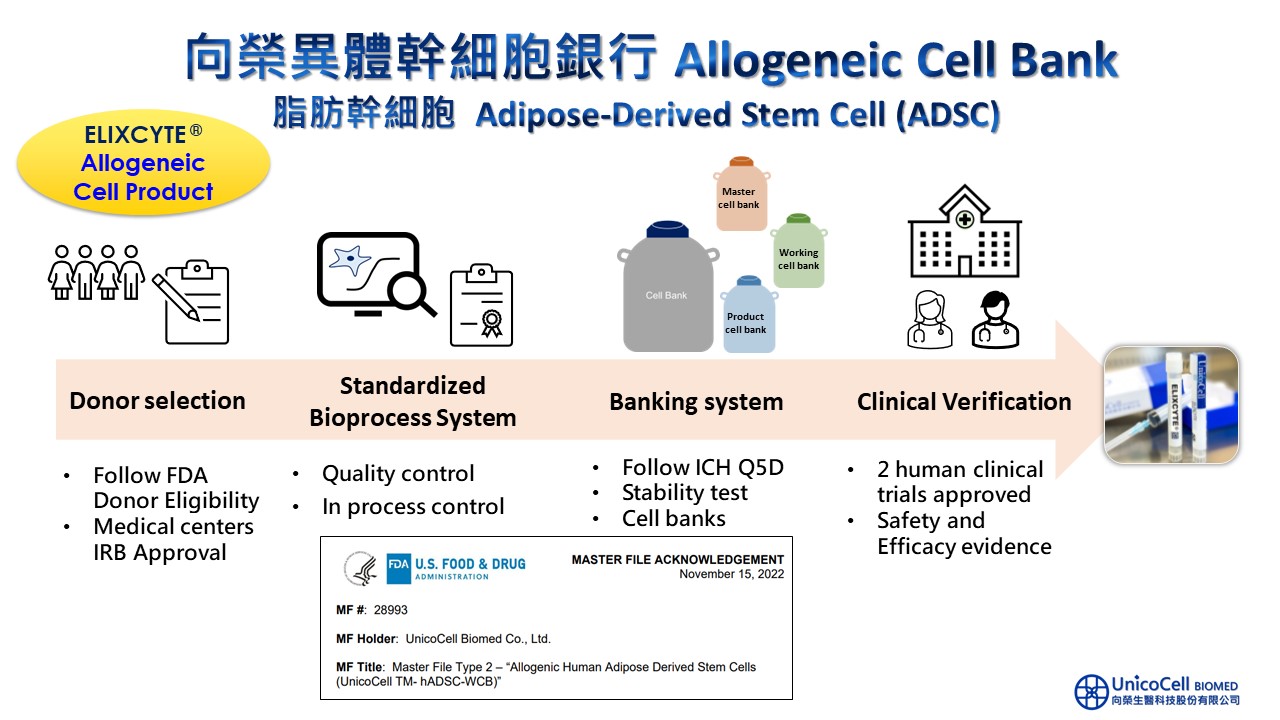
UnicoCell's adipose-derived stem cell (ADSC) allogeneic cell bank stands as the only cell storage facility in Taiwan accredited by the U.S. FDA's Master File. From donor eligibility to the establishment of the cell bank, UnicoCell strictly followed FDA and TFDA regulations, including multiple viral and safety tests.
UnicoCell allogeneic cell bank was established in compliance with International Council for Harmonisation (ICH) requirements. In addition to satisfying the clinical trial development demands for novel pharmaceuticals, the UnicoCell allogeneic cell bank is the finest choice for cell starting materials and can provide an industry-university research unit that carries out the development of various indications.
The stem cell product ELIXCYTE®, cultivated from UnicoCell allogeneic cell bank, is currently undergoing Phase III clinical trials for knee osteoarthritis. Additionally, the Phase II clinical trials for chronic kidney disease have been completed and are now in the data analysis stage. ELIXCYTE®, by UnicoCell, not only maintains stringent control over its raw material sources but also boasts a rigorous quality management system. Its safety and efficacy have been recognized by the U.S. FDA's Master File, positioning it for a streamlined drug approval process in the future. These outstanding attributes solidify UnicoCell allogeneic cell bank as a premier source for clinical trials and academic research.
Features / strengths
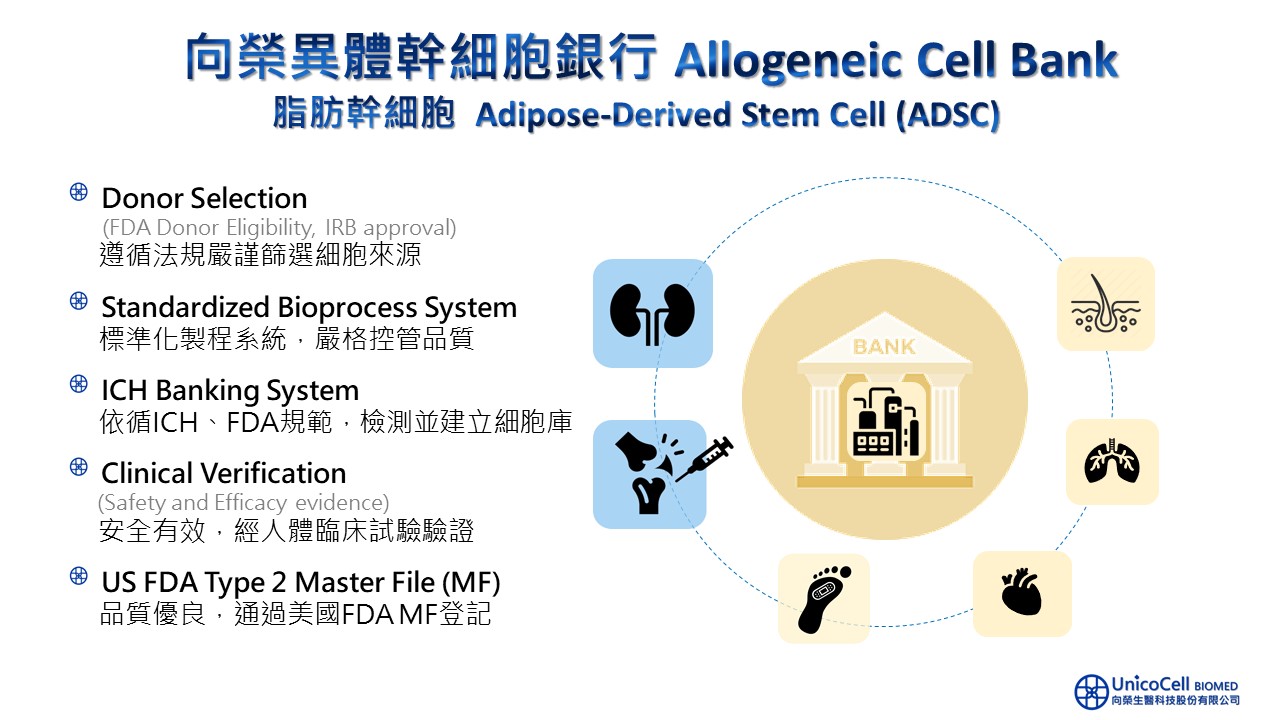
˙ The only one in Taiwan to receive the U.S. FDA's Master File approval.
˙ From donor qualification to cell bank establishment, all processes adhere to ICH guidelines.
˙ Cell source and quality have been validated through clinical trials and scientific publications.
˙ Passed comprehensive safety and viral testing.
Specification in detail
Content
Human adipose-derived stem cell