Introduction
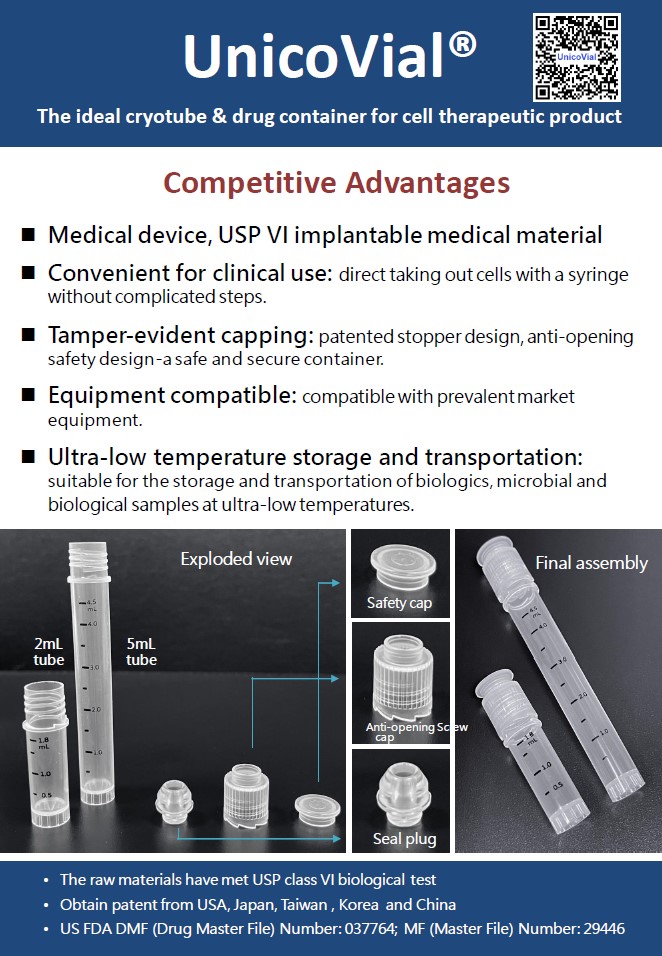
Contrary to the current biotech industry which predominantly uses non-medical grade research vials for storage, UnicoCell has developed the world's unique ultra-low temperature biological preparation storage container, UnicoVial®. Not only did it receive a Class II medical device approval from Taiwan's Ministry of Health and Welfare in 2021, but it also secured the U.S. FDA's Master File (MF) and Drug Master File (DMF) registration in 2023.
Features / strengths
˙ It is the only cell storage container approved as a Class II medical device for ultra-low temperatures in Taiwan.
˙ Obtain patent from USA, Japan, Taiwan , Korea and China
˙ Received Acknowledgment letter of DMF (Drug Master File) and MF (Master File) from US FDA
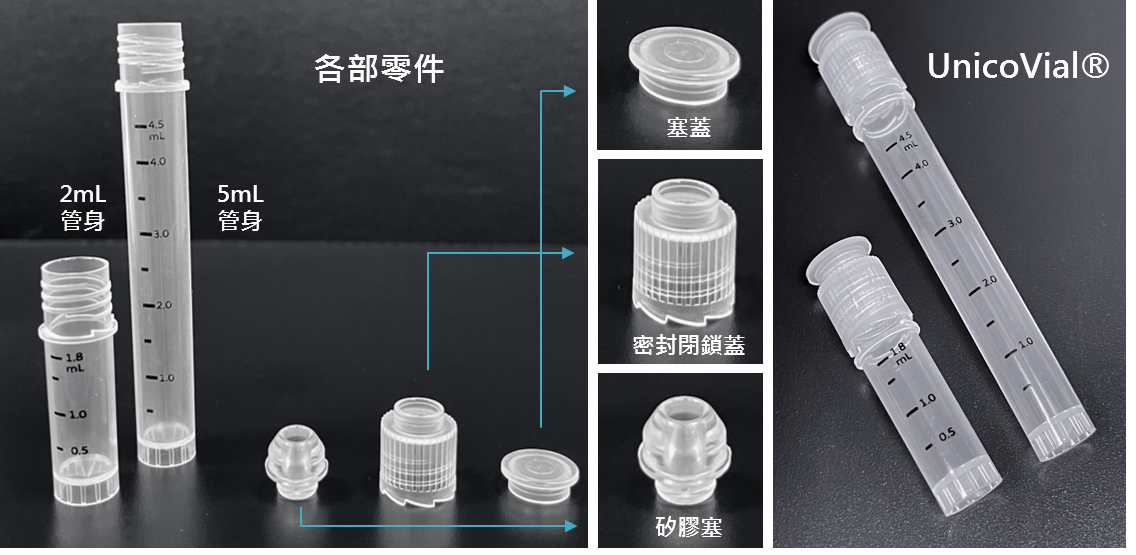
˙ Features a patented sealed anti-opening design, fully compliant with clinical safety usage standards.
˙ Compatible design with commonly used equipment on the market; no specific binding specifications required.
˙ Ensures long-term stability at ultra-low temperatures, making it suitable for preserving cells and biologics in extreme cold conditions.
Specification in detail
Certification
Class II medical device
Supporting document