Introduction
ABCcolla® Bone Graft is a decellularized native porcine bone graft material prepared by supercritical CO2 extraction technology, which is composed of native porcine collagen and bone matrix that are comparable to the human bone matrix. ABCcolla® Bone Graft is intended for filling and augmentation of bone voids or gaps during orthopedic surgeries. It facilitates cell attachment and improves bone regeneration and neovascularization. During the healing process, ABCcolla® Bone Graft gradually resorbs and is replenished by new bone.
----
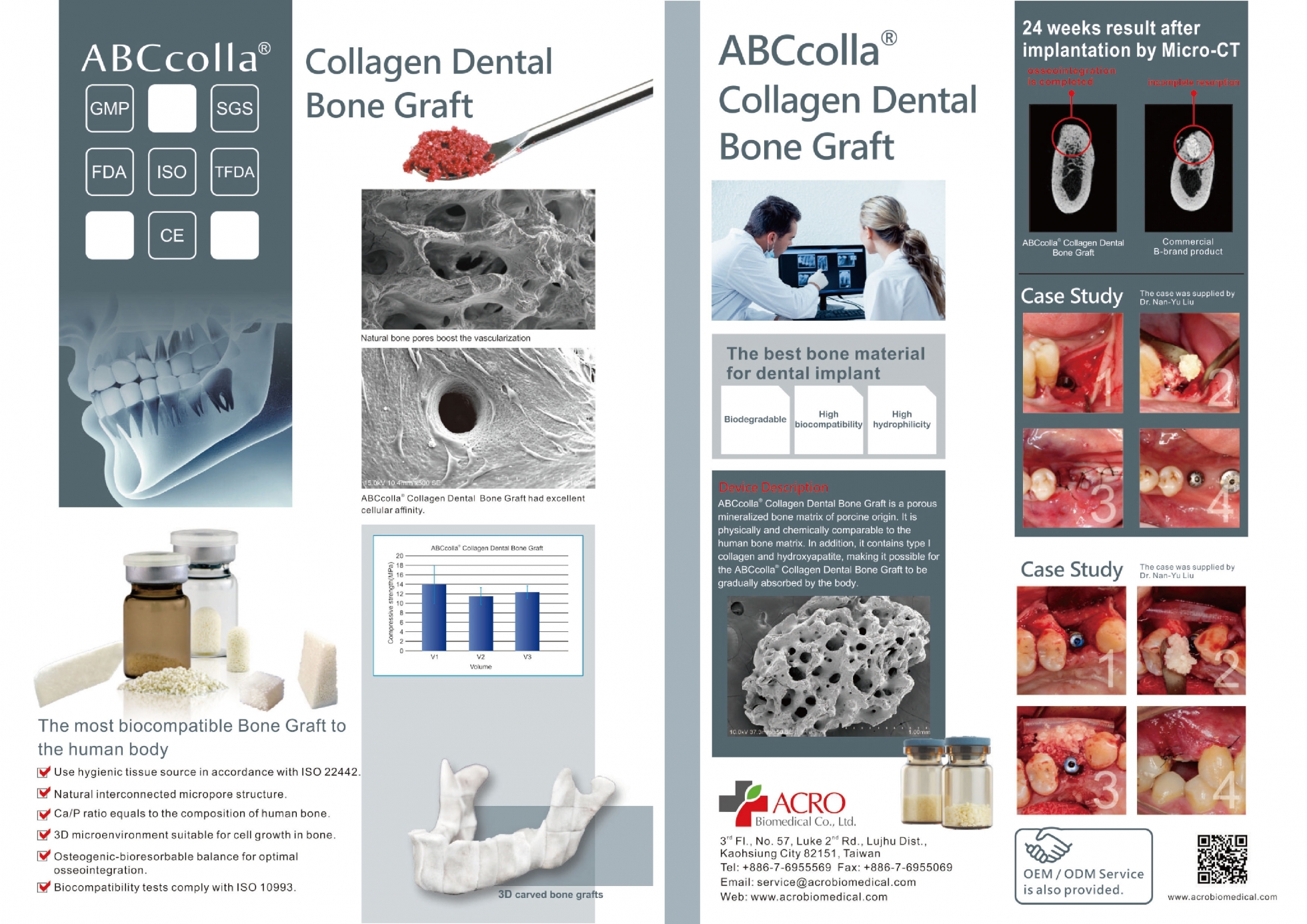
ABCcolla® Collagen Dental Bone Graft is a decellularized native porcine bone graft material prepared by supercritical CO2 extraction technology, which is composed of native porcine collagen and bone matrix that are comparable to the human bone matrix. ABCcolla® Collagen Dental Bone Graft is indicated to be filled into bony voids or gaps and augment oral, dental intraosseous, and craniofacial defects. These defects may include: periodontal/infrabony defects, alveolar ridge augmentation (sinusotomy, osteotomy, cystectomy), dental extraction sites (ridge maintenance, implant preparation/placement), sinus lifts, cystic defects, and craniofacial augmentation. ABCcolla® Collagen Dental Bone Graft may be used alone in a manner comparable to autogenous bone graft chips or allograft bone particulate (demineralized freeze-dried bone), or it may be mixed with either as a bone graft extender.
Indication:
Augmentation or reconstructive treatment of the extraction sockets.
Filling of voids after root resection.
Filling of extraction sockets to enhance preservation of alveolar ridge.
Filling of extraction sockets in conjunction with products intended for Guided Tissue Regeneration (GTR) and Guided Bone Regeneration (GBR).
Features / strengths
*No BSE risk.
*Natural bonal pores for neovascularization.
*Natural mineral contains for better osteoreconstruction.
*Excellent biocompatibility and hydroaffinity.
Specification in detail
Specifications
granule, cone, cube
Volume
0.5 c.c., 1.0 c.c., 2.0 c.c.
Particle size
0.25-1.0mm, 1.0-2.0mm
Cone size
ø10mmx10mm, ø10mmx15mm
Cube size
10x10x10 mm, 5x5x5 mm
Medical Device Certificates
Taiwan(TFDA), US(FDA 510K), Singapore, Vietnam, the Philippines