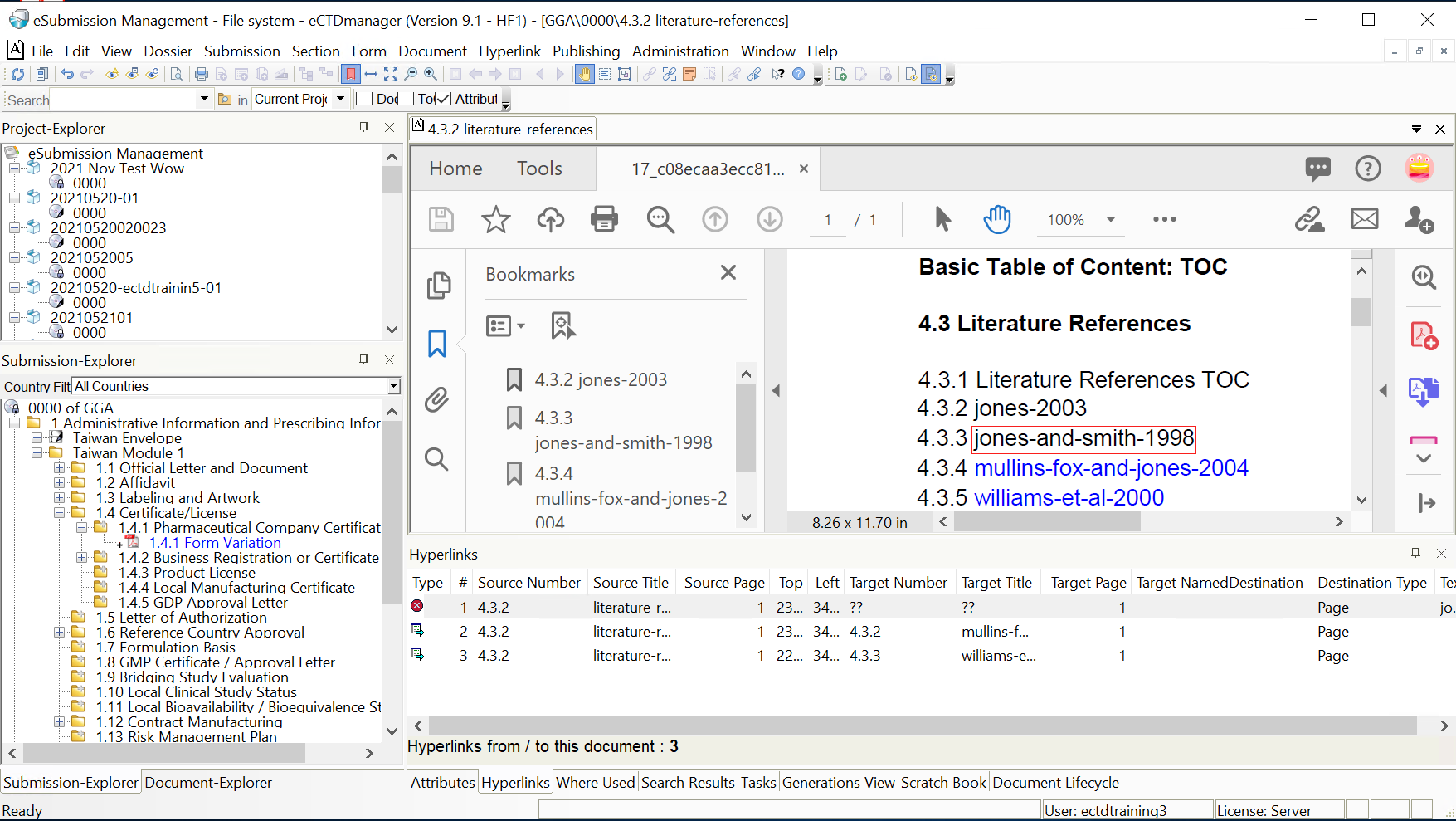
Introduction
Creating and maintaining submissions that are compliant with global regulatory standards is complex and time-consuming. EXTEDO eCTDmanager is the most comprehensive eCTD management solution, simplifying your submission management. From creating, publishing to ongoing maintenance, eCTDmanager streamlines end-to-end submission processes, enabling you to generate error-free, compliant submissions. It can create, review, validate, and publish compliant submissions in various formats, including eCTD, NeeS, ACTD, VNeeS, ASMF, and other regional formats. Its powerful hyperlinks and bookmark engine allow the detection and notification of broken links, thereby enhancing the quality and consistency of submissions. Built-in technical validation ensures that submission is valid according to the latest authorities validation standards. The intuitive interface enables you to easily handle electronic submissions without prior knowledge of XML technology, and its unique visual aids provide context, ensuring a smooth and unprecedented level of accuracy in the process. Integrated with the DOCmanager module as an add-on to eCTDmanager, any changes you make to parent dossiers are automatically inherited by child dossier. This eliminates the need for revalidation and thereby, reducing the workload for file editing and maintaining for multi-regional product dossiers.
Features / strengths
●Built-in validation functiona
●Compatible with multiple electronic submission formats: eCTD, NeeS, and more
●Integratable with Document Management Systems (DMS)
●Compliant with US FDA CFR Part 11 and ISO/IEC 27001
Specification in detail
Create, review, validate, publish, and search.
Submission formats
eCTD, NeeS, ACTD, VNeeS, DMF, ASMF, US-MedDev and other regional formats
eCTD submission countries
EU, US, AU, CA, CH, GCC, JO, JP, TH, ZA, EAEU, CN, TW