Introduction
A solution for poor solubility, extended release, depot formulation, and abuse deterrence
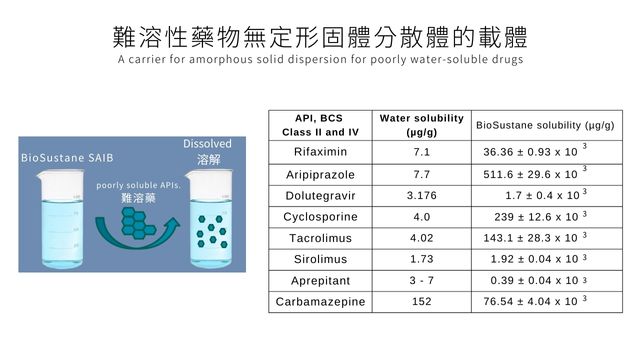
Highly hydrophobic Eastman BioSustane™ SAIB NF can be used as a carrier for amorphous solid dispersions.
This novel excipient does not need hot-melt extrusion or spray-drying manufacturing techniques.
Traditional wet granulation techniques can be used to formulate BioSustane amorphous solid dispersions into tablets or capsules.
When used as a carrier, BioSustane is shown to improve bioavailability by preventing recrystallization of poorly water-soluble APIs.
Features / strengths
‧A carrier for amorphous solid dispersion for poorly water-soluble drugs,
‧ improve bioavailability by preventing recrystallization of poorly water-soluble APIs.
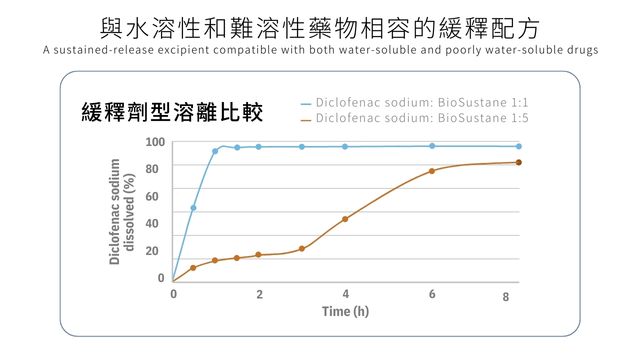
‧A sustained-release excipient compatible with both water-soluble and poorly water-soluble drugs
‧improves the bioavailability of Aprepitant in beagles
‧A novel depot former for injectable drugs as shown in some U.S. Food and Drug Administration (FDA) approved drug formulations. Note: Eastman does not market an injectable grade of BioSustane.
Specification in detail
Welcome to contact us to order a sample or talk to an expert.