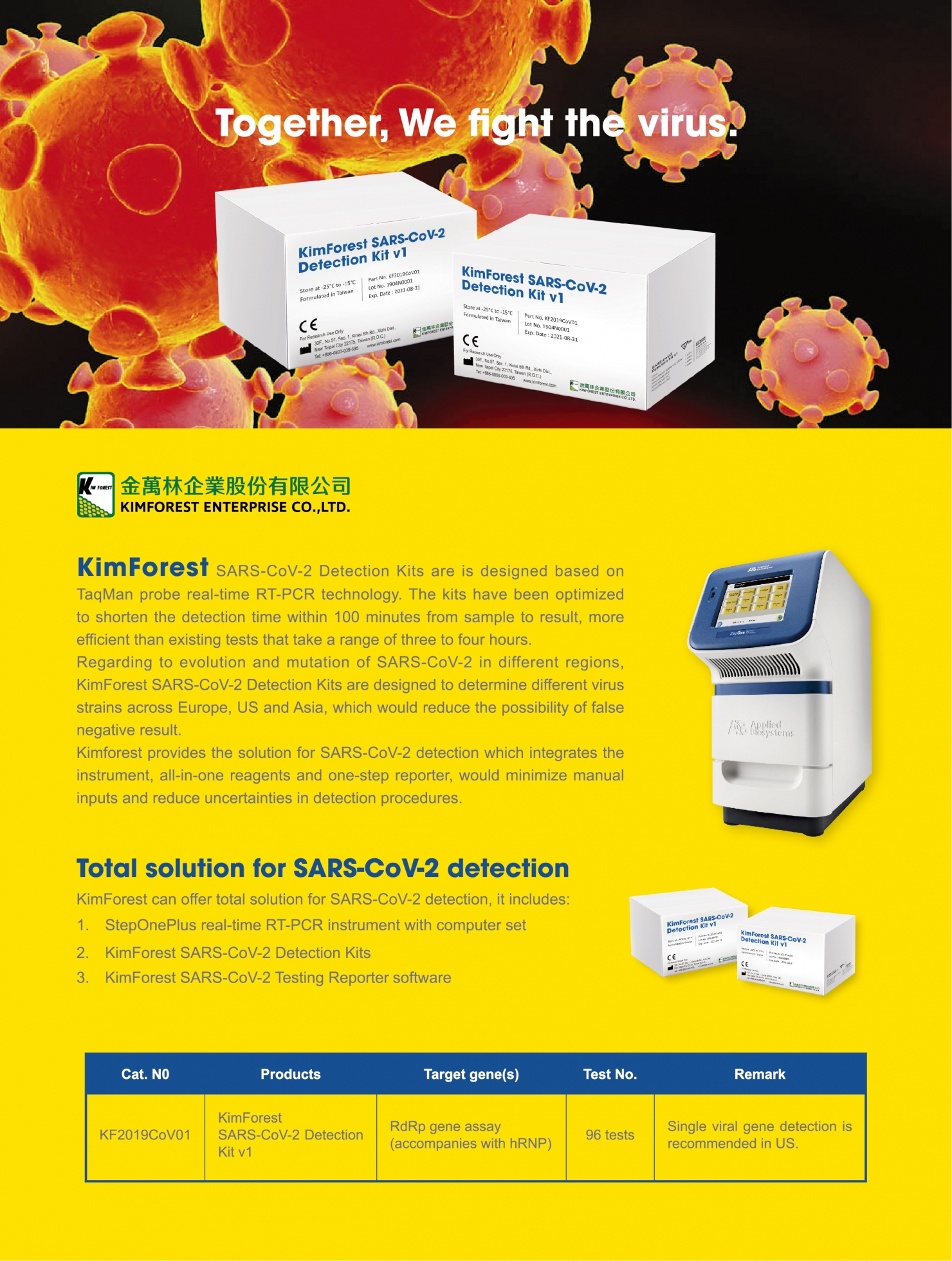
Introduction
KimForest_Certificate list:
◆ TFDA Production Certificate
◆ TFDA Export Certificate
◆ Certificate of Origin
◆ US FDA EUA Manufacturer Certificate
◆ US FDA EUA Laboratories Notification- PacGenomics
◆ US FDA EUA Laboratories Notification- Sun Clinical Lab
◆ Republic of the Philippines FDA Certificate
◆ CE EAR Certificate
◆ CE Notification
Features / strengths
☑ Time-saving
50 mins, for real-time RT-PCR detection
☑ User-friendly analysis software
15 mins, getting 94 induvial reports automatically
☑ Quick testing flow
2 hours, from sample collection to repot
☑ High sensitivity
SARS-CoV-2 isolates across all continents detectable
by KimForest Kit in silico analysis
☑ High specificity
No cross reactivity with common respiratory flora
and other viral pathogens
Specification in detail
Quantity
100μl、500μl、50μl、1ml
Function
SARS-Cov2 viral RNA quantitation
Supporting document