Introduction
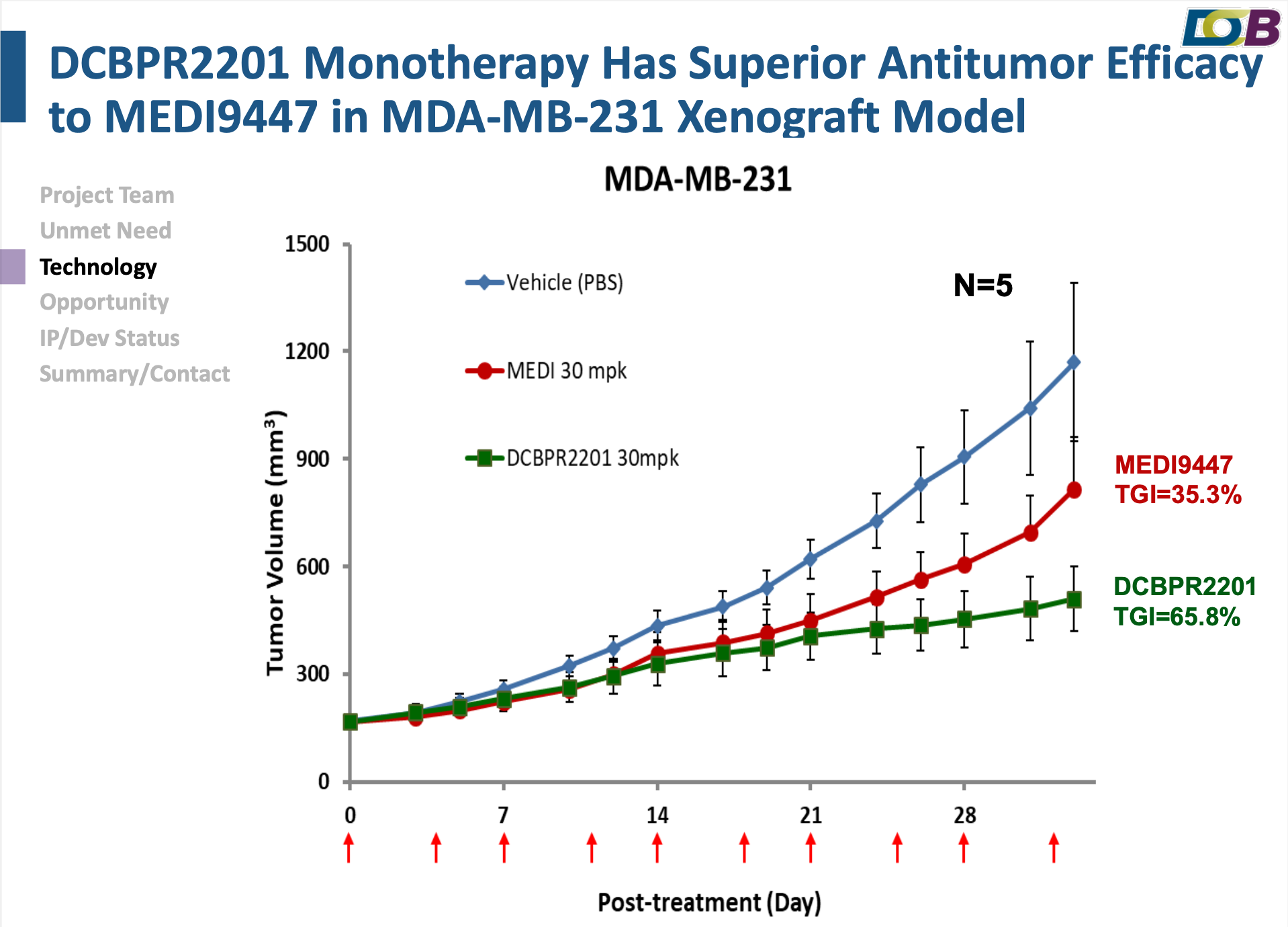
In the tumor microenvironment, the high expression of CD73 results in the increase of Adenosine, which inhibits the activation of immune cells. Therefore, anti-CD73 antibodies that efficiently inhibits CD73 enzyme activity, is able to reduce the production of Adenosine and restore the function of immune cells. Anti-CD73 antibodies can be used in combination with existing anti-cancer therapies to improve the efficacy of current treatment. Herein, DCB has developed a novel humanized anti-CD73 candidate antibody DCBPR2201 with novel CDR sequence and binding epitope. In biological activity aspect, DCBPR2201 exhibits long-lasting inhibition ability against cell surface CD73, significantly reverses AMP's suppressed T cell activation, and effectively inhibits intra-tumoral CD73 activity as well as tumor growth in CD73 relevant MDA-MB-231 TNBC xenograft model. The overall in vitro and in vivo activities of DCB’s CD73 antibody are better than competitor MEDI9447, demonstrating the international competitiveness and drug developmental potential. The regular patent has been applied in PCT, US and Taiwan.
Features / strengths
✓ Unique CDR sequences and epitopes
✓ Long-lasting inhibition against CD73 activity
✓ Superior in vitro and in vivo activities to benchmark MEDI9447
✓ High safety in NHP DRF study
✓ Cancer immunotherapy/Targeting CD73 highly expressed cancer e.g. lung cancer & pancreatic cancer
Specification in detail
Development of Anti-CD73 Antibody for Cancer Immunotherapy
Humanized CD73 antibody with high potency and safety